Senior CQV Engineer for pharmaceutical projects throughout Belgium
on clients' site throughout Belgium
Contract :
Permanent or Freelance
Schedule :
Full-time
Organisation size :
11-50
The Scope
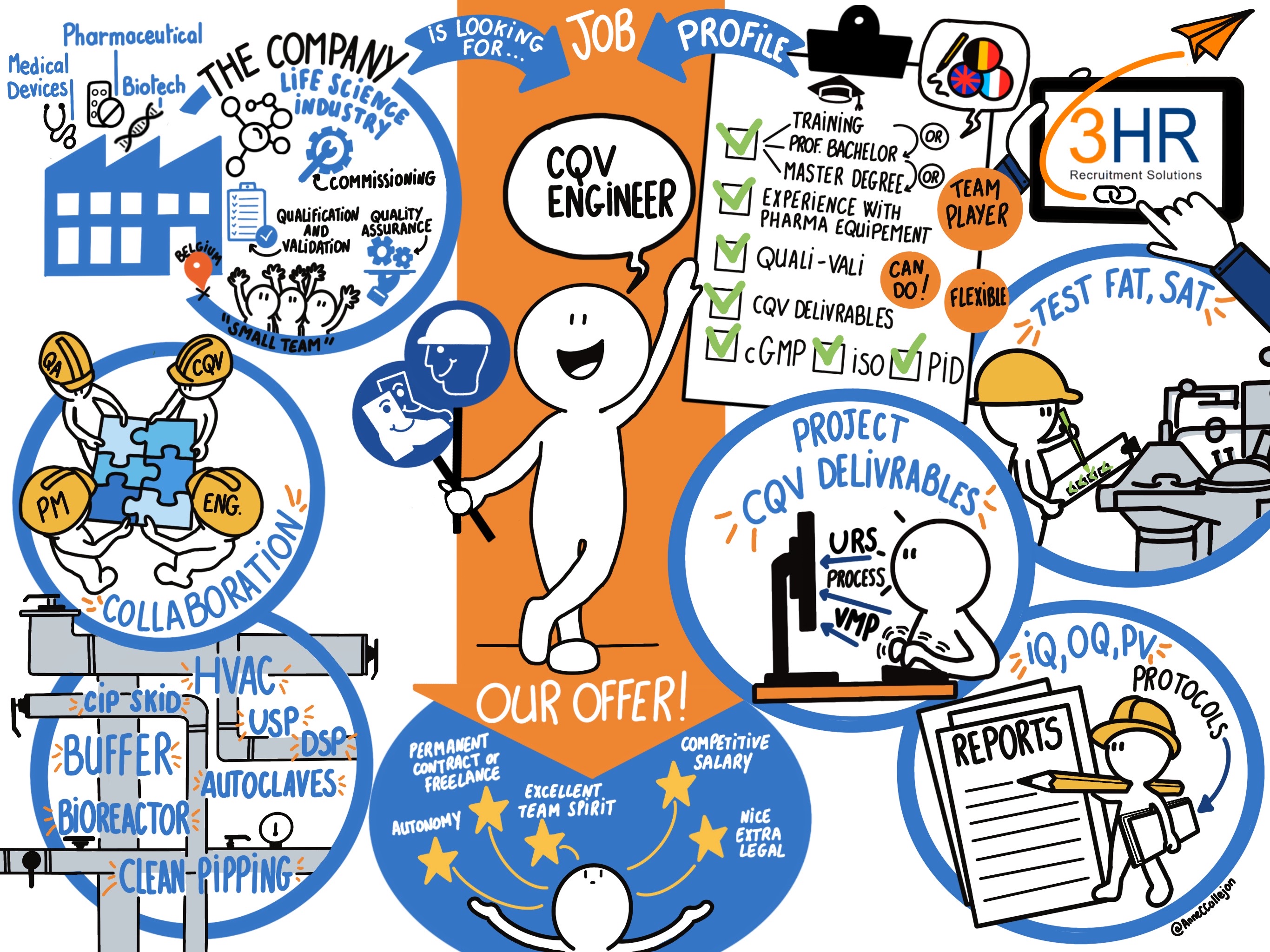
3HR.be is the Recruitment Partner of Entrepreneurs & small companies, do you like working in such a business with a family ambiance?
One of our client is a Belgian Engineering office active in various Life Science Industries like Pharmaceutical, Biotechnology, Medical Devices, with expertise in Qualification & Validation, Quality Assurance, Consulting, Engineering and Construction Management.
With a constant evolving team composed 30 employees & freelances and offices in Belgium, this client is throughout the year looking for Senior Commissioning, Qualification, Validation Engineers for pharmaceutical projects (on site) throughout Belgium.
Are you ready to contribute to their growth?
Do you have an entrepreneurial mindset and are you passionate about working in Life Science industries?
We love meeting smart and forward-thinking talents, so don’t hold back!
Your key tasks and responsibilities
As one of their Senior CQV Engineers, you will
- Have a knowledge of current Industry standards, regulatory requirements and Authority expectations regarding CQV
- Develop project specific CQV approach and methodology
- Prepare project CQV deliverables:
- User Requirement Specifications
- Risk and Impact Analysis/Assessment (Process, Business, EHS, Regulatory)
- Validation Master Plan (VMP)
- Design Review/Qualification
- Test Matrices
- FAT, SAT, Commissioning, IQ, OQ, PQ protocols and reports
- Support in project schedule for CQV activities
- Organise, execute and document CQV testing
- Support during system/installation start-up in function of CQV test activities
- Set up and follow up of Project documentation System and Management
- Work in close collaboration with Project, Engineering and Construction Management
- Be in charge of the registration of quality indicators for CQV Engineer’s discipline
You could be our ideal candidate if
- You hold a (Professional) Bachelor or a Master’s degree (or equivalent by experience), ideally in biotechnology, industrial, pharma, chemical sciences or relevant other expertise
- You have a significant experience (min. 4 years) in Life Sciences industries
- You have excellent communication skills in English + French OR Dutch (written and spoken)
- You have an enhanced knowledge of current Industry Standards, Regulatory requirements and Authority expectations (Eudralex, FDA, PICS, ICH, ISO, ASTME2500 …)
- You have a good Comprehension and understanding of technical installation, PID, equipment and buildings for the Life Science Industry
- You know the latest developments in the C&Q field and relevant legislation and industry standards (cGMP).
- You have a field experience with:
- System start-up and CQV
- Project scheduling
- Change management
- Project document management
- You are a structured, conscientious and precise fast-learner
- You are a real team player who can also work independently
- You are ready to travel and you are flexible on schedules and duties
- You are resourceful with a CAN DO mentality who can think proactively
- You have got a B driving licence
- ⚠ Only Europe-based and selected candidates will be contacted back by 3HR, thanks for your understanding.
We offer you
Besides an exciting flexible environment, a unique chance to participate in challenging and innovative projects and interaction with inspiring colleagues who have a professional attitude and attach considerable importance to teamwork; we stand for
- A challenging job in a dynamic and knowledge-sharing network company
- Full time contract
- Location: Flexible depending to your mobility and clients’ locations
- A competitive salary or rate
- Other benefits for employees including a company car, fuel card, lunch vouchers, eco vouchers, pension plan, phone subscription
- The freedom to develop yourself in line with your ambition
- A great autonomy in your role, an excellent working atmosphere within a great team of Experts in their domain
Would you like to work for a small company with a top level service?
We are looking forward to meeting you soon.
Your application
We’re looking for motivated and passionate individuals who love to build things and solve problems.
If this is you, please apply here addressing your application, CV and a short motivation text, to Mrs. Gwendoline de Robiano
⚠ Only Europe-based and selected candidates will be contacted back by 3HR Recruitment Solutions, thanks for your understanding.
